How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora
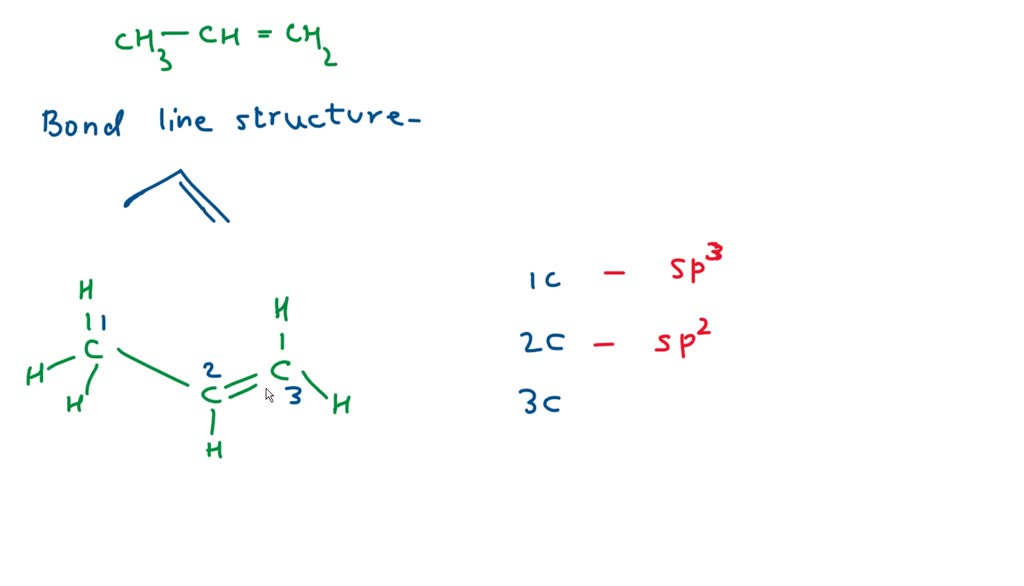
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...
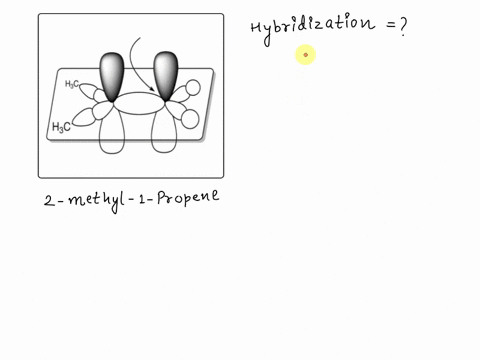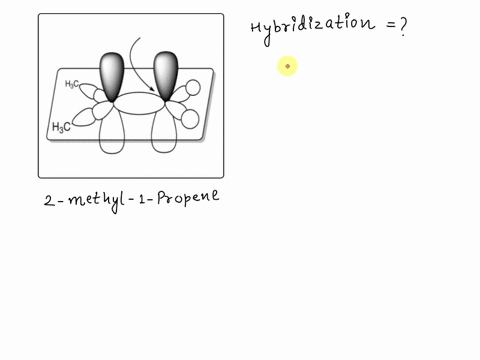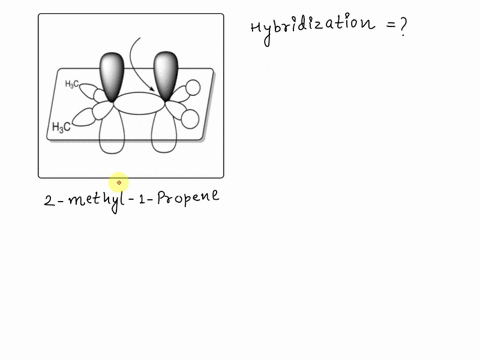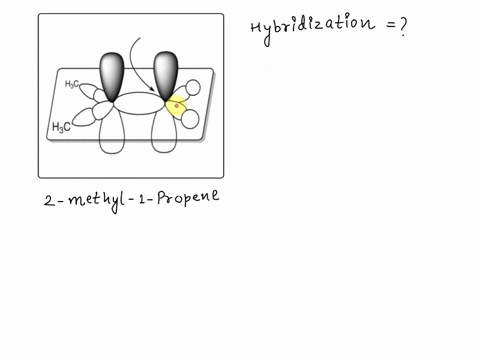
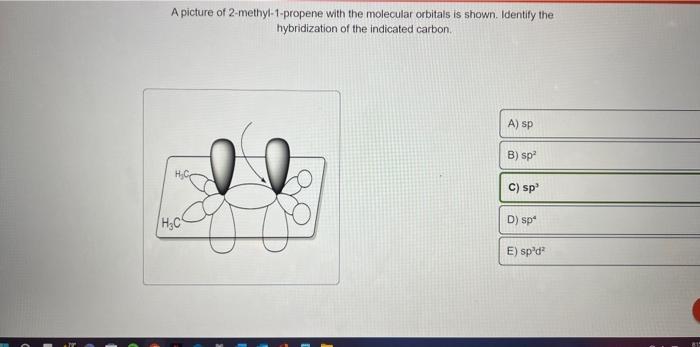
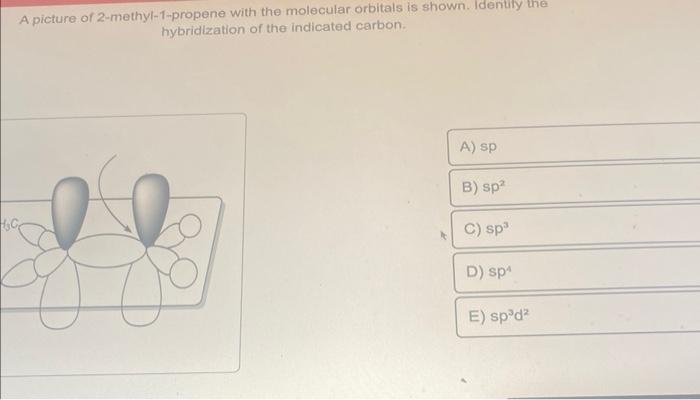
SOLVED: Texts: A picture of 2-methyl-1-propene with the molecular orbitals is shown. Identify the hybridization of the indicated carbon. A) sp B) sp2 C) sp D) sp3 E) sp2
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...

Propene C3H6: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure – infographic

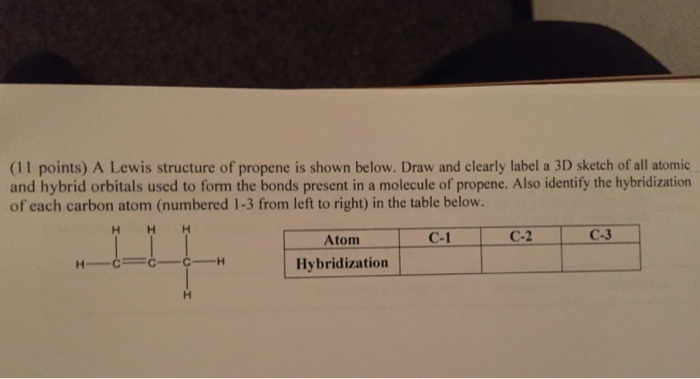
Draw the molecular shape of propene and determine the hybridization of the carbon atoms. Indicate which orbitals overlap with each other to form the bonds. | Homework.Study.com
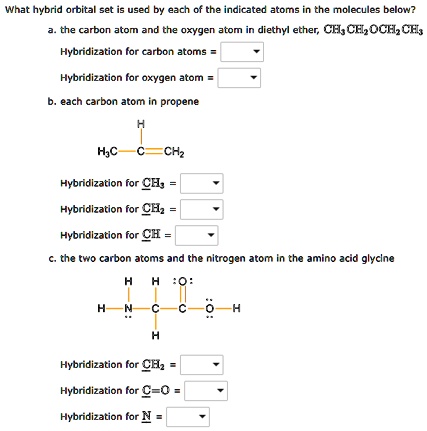
SOLVED: Text: What hybrid orbital set is used by each of the indicated atoms in the molecules below? a. the carbon atom and the oxygen atom in diethyl ether, CH3COCH2CH3 Hybridization for
In the displayed formula of propene, why are the two hydrogens on the right, drawn at an angle? Do I need to replicate these angles when draw the displayed formula of propene? -

Orbital Hybridization Practice Problems - Organic Chemistry #stemeducation #stem #chemistry - YouTube

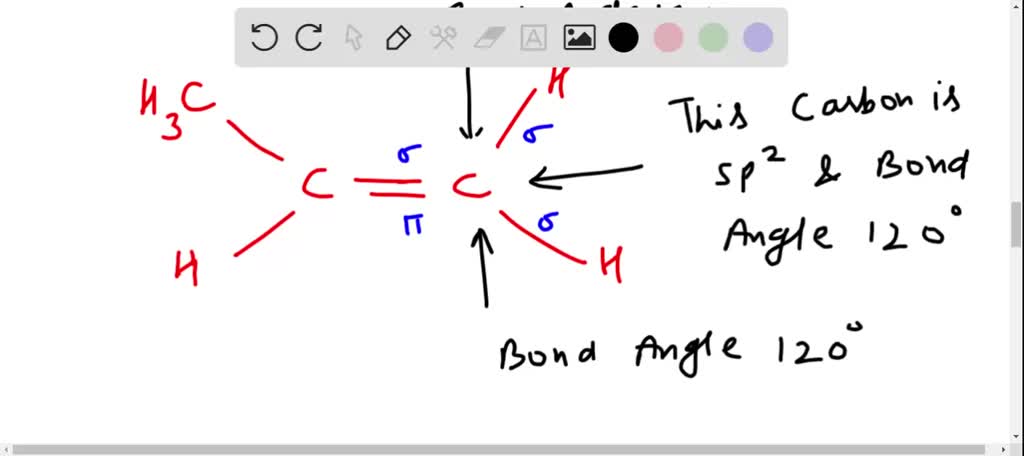
How exactly is this carbon considered sp2 hybridized when it has 3 substituents and a lone pair? : r/OrganicChemistry

Scheme 3. Conformations of propene in the s-p representation (3, 4)a nd... | Download Scientific Diagram