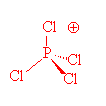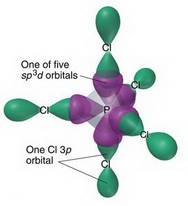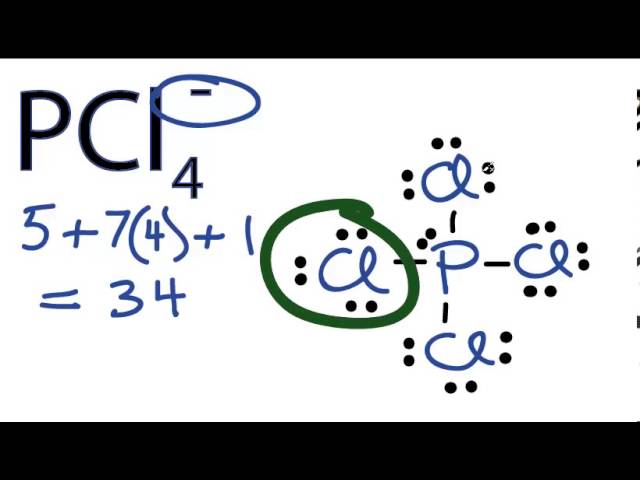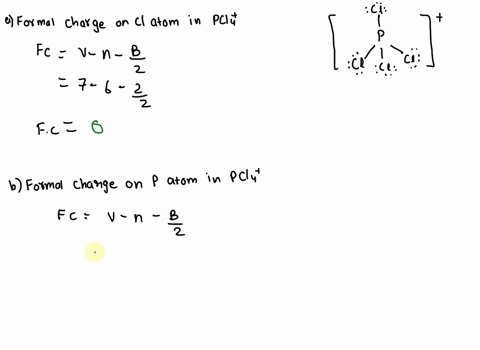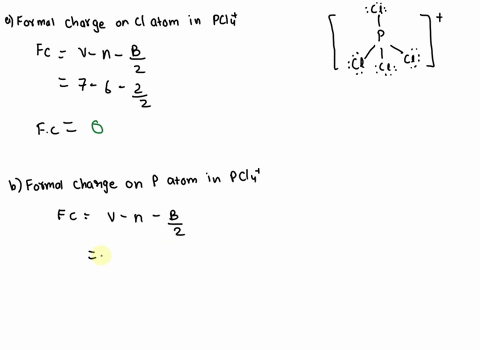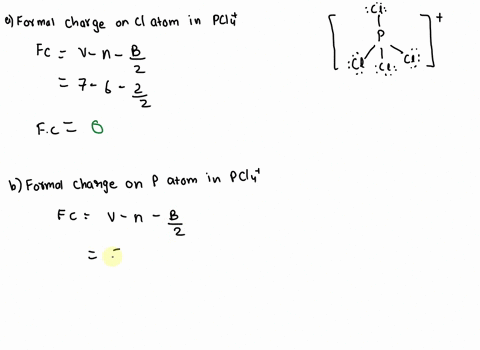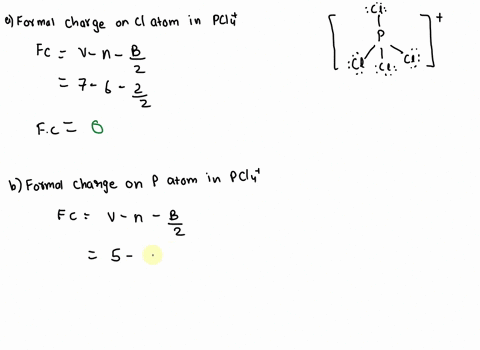
SOLVED: 2. What is the formal charge on the phosphorus (P) atom in PCl4+? 3. How do the P-Ci single bond lengths in PCl5, PCl4+, and PCl6- generally compare? 4. Identify the
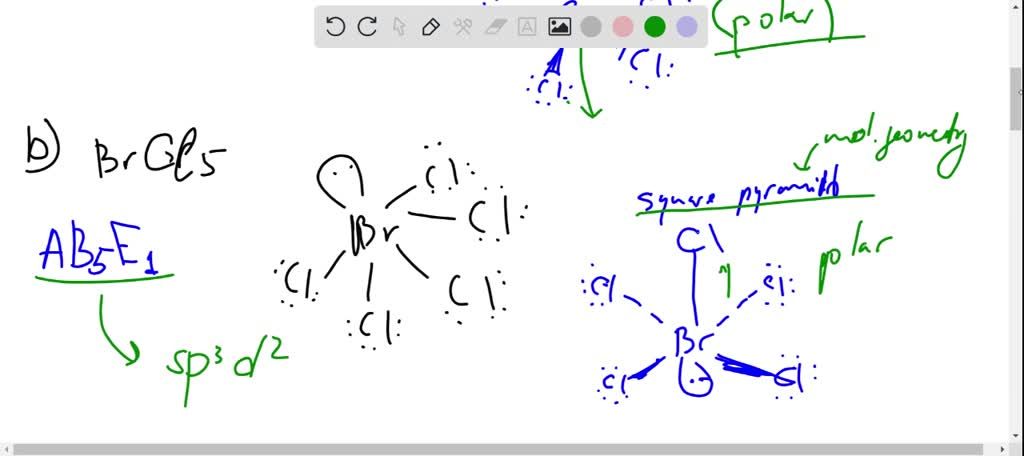
SOLVED: For each of the following molecules: (i) draw the correct Lewis structure; (ii) determine the molecular geometry and the type of hybridization on the central atom, and (iii) predict whether the

The shapes of PCl^+_4,, P{ Cl }^-_4 and AsCl_5 are respectively:tetrahedral, see-saw and trigonal bipyramidalsquare planar, tetrahedral and see-sawtetrahedral, square planar and pentagonal bipyramidaltrigonal bipyramidal, tetrahedral and square pyramidal

Consider the phosphorus tetrachloryl (PCl4+) cation. How many lone pairs are around the central atom? | Homework.Study.com

The shapes displaystyle:PCl_{4}^{+}, PCl_{4}^{-} and displaystyle:AsCl_{5} are respectively:square planar, tetrahedral and see-sawtetrahedral, see-saw and trigonal bipyramidaltetrahedral, square planar and pentagonal bipyramidaltrigonal bipyramidal ...
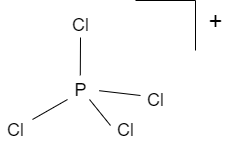
The shapes $PCl_{4}^{+},PCl_{4}^{-}$ and $AsC{{l}_{5}}$ are respectively:A. square planar, tetrahedral and see-sawB. tetrahedral, see-saw and trigonal bipyramidalC. tetrahedral, square planar and pentagonal bipyramidalD. trigonal bipyramidal ...
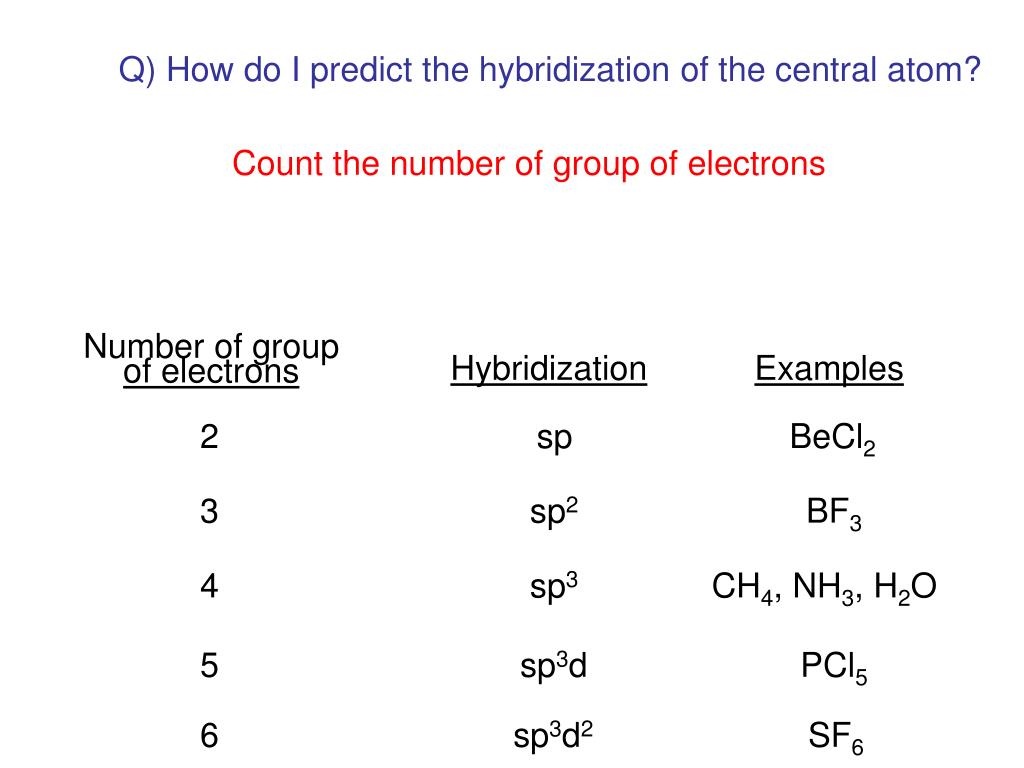
PPT - Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals PowerPoint Presentation - ID:5587869

PCl4+ (Phosphorus tetrachloryl ion) Molecular Geometry, Bond Angles (and Electron Geometry) - YouTube

Which of the following has a bond angle of approximately 120 deg? a) ClF3 b) SbBr6- c) PCl4- d) BeCl2 | Homework.Study.com

Consider the phosphorus tetrachloryl (PCl4+) cation. What is the central atom? Enter its chemical symbol. | Homework.Study.com

PCl4+ (Phosphorus tetrachloryl ion) Molecular Geometry, Bond Angles (and Electron Geometry) - YouTube