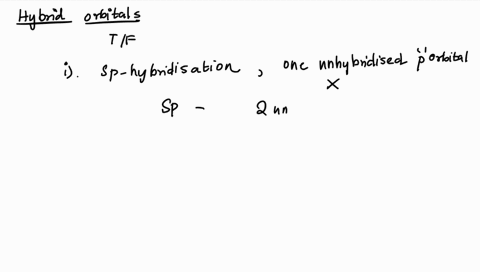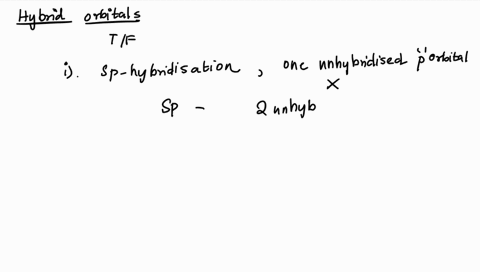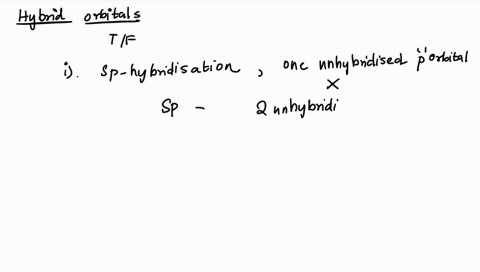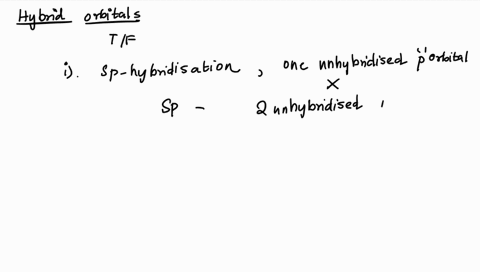
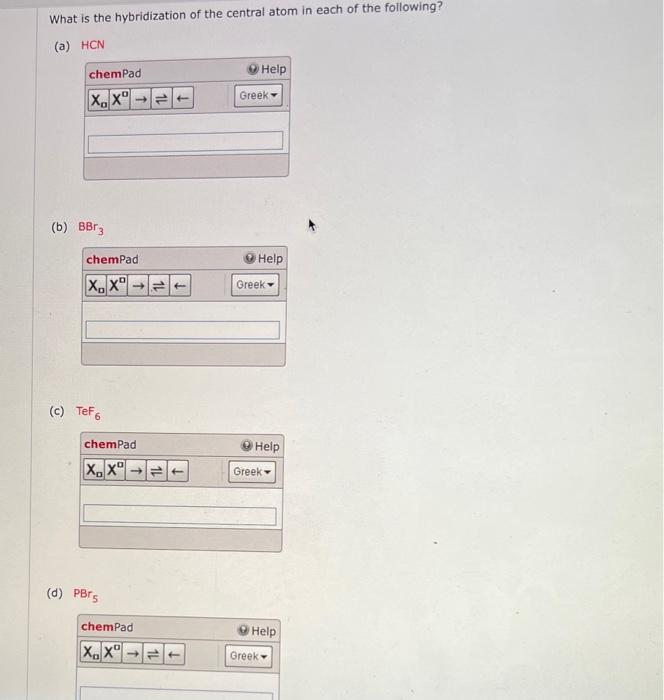
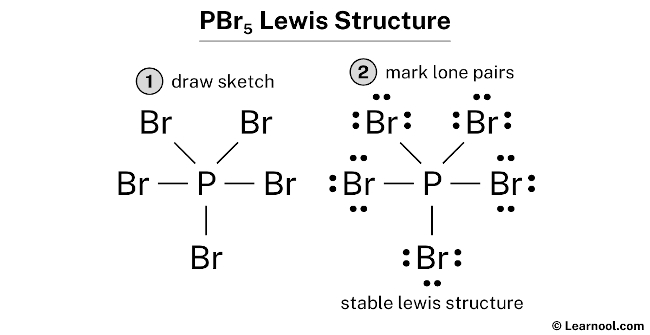
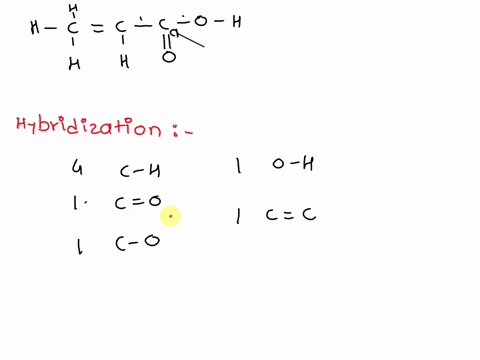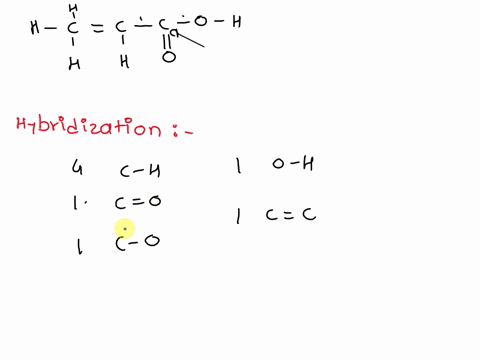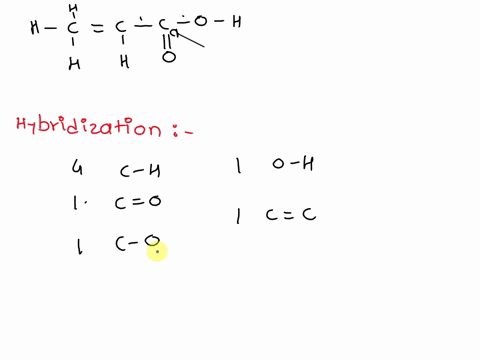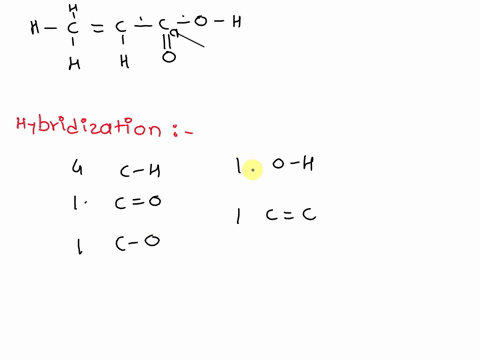
1. Explain the hybridisation and geometry of the following molecules. a. PBr5 b. ClF3 c. SF4 d. PH3 e. - Brainly.in

Types of Hybridization: Definitions, Examples, Key Features, Steps to Determine, Shapes, and Rules | CollegeSearch
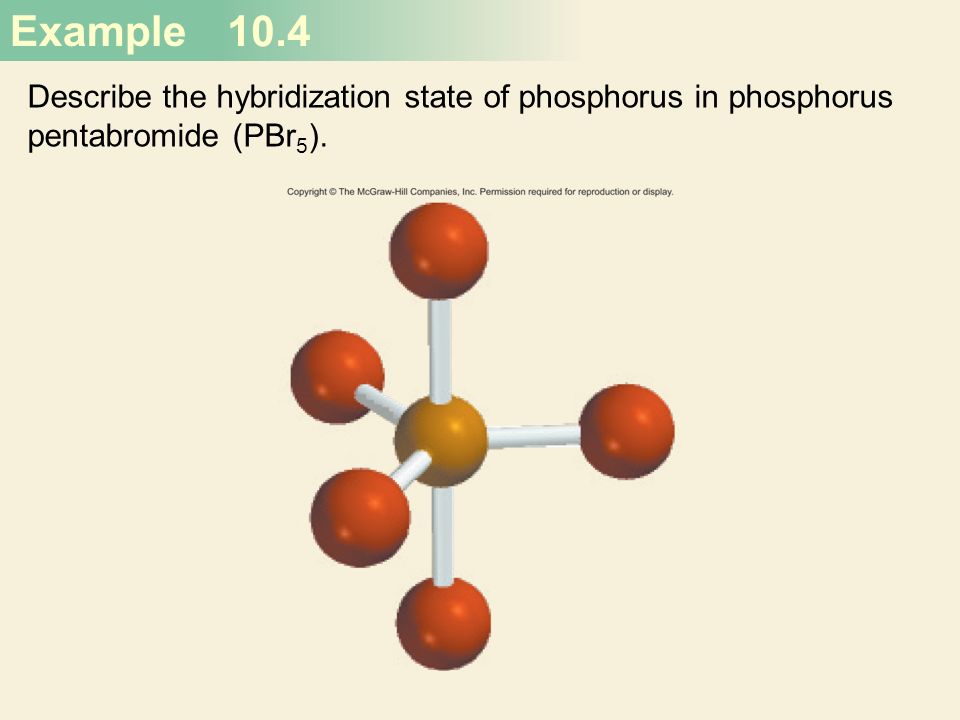
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter ppt video online download

What are the electron geometry and molecular geometry around the central atom of phosphorus pentabromide? | Homework.Study.com

why PBr5 as sp3 hybridization not sp3d hybridization - Chemistry - Chemical Bonding and Molecular Structure - 13043843 | Meritnation.com

12. Which of the following compounds exhibit d 2sp 3 hybridization? (Select all that apply.) BrF5 ClF5 KrCl4 XeCl2 PCl5 13. Draw the Lewis structure for IF3 and answer the following question.

12. Which of the following compounds exhibit d 2sp 3 hybridization? (Select all that apply.) BrF5 ClF5 KrCl4 XeCl2 PCl5 13. Draw the Lewis structure for IF3 and answer the following question.