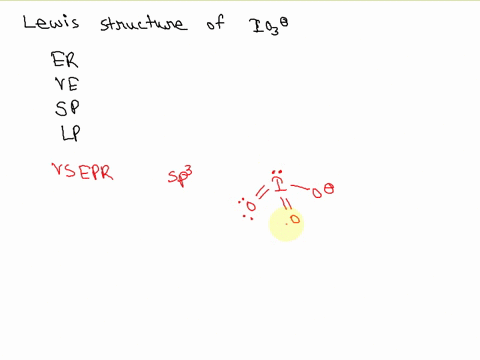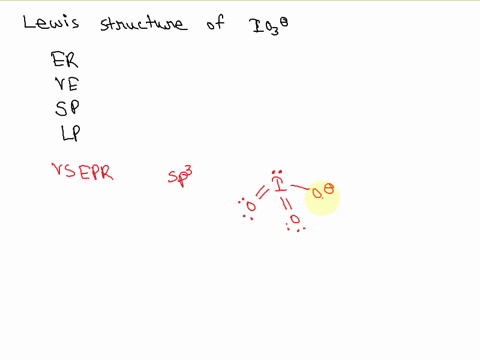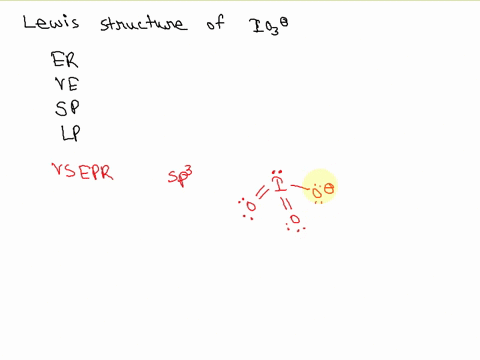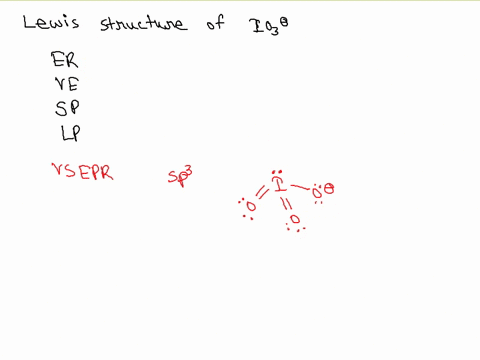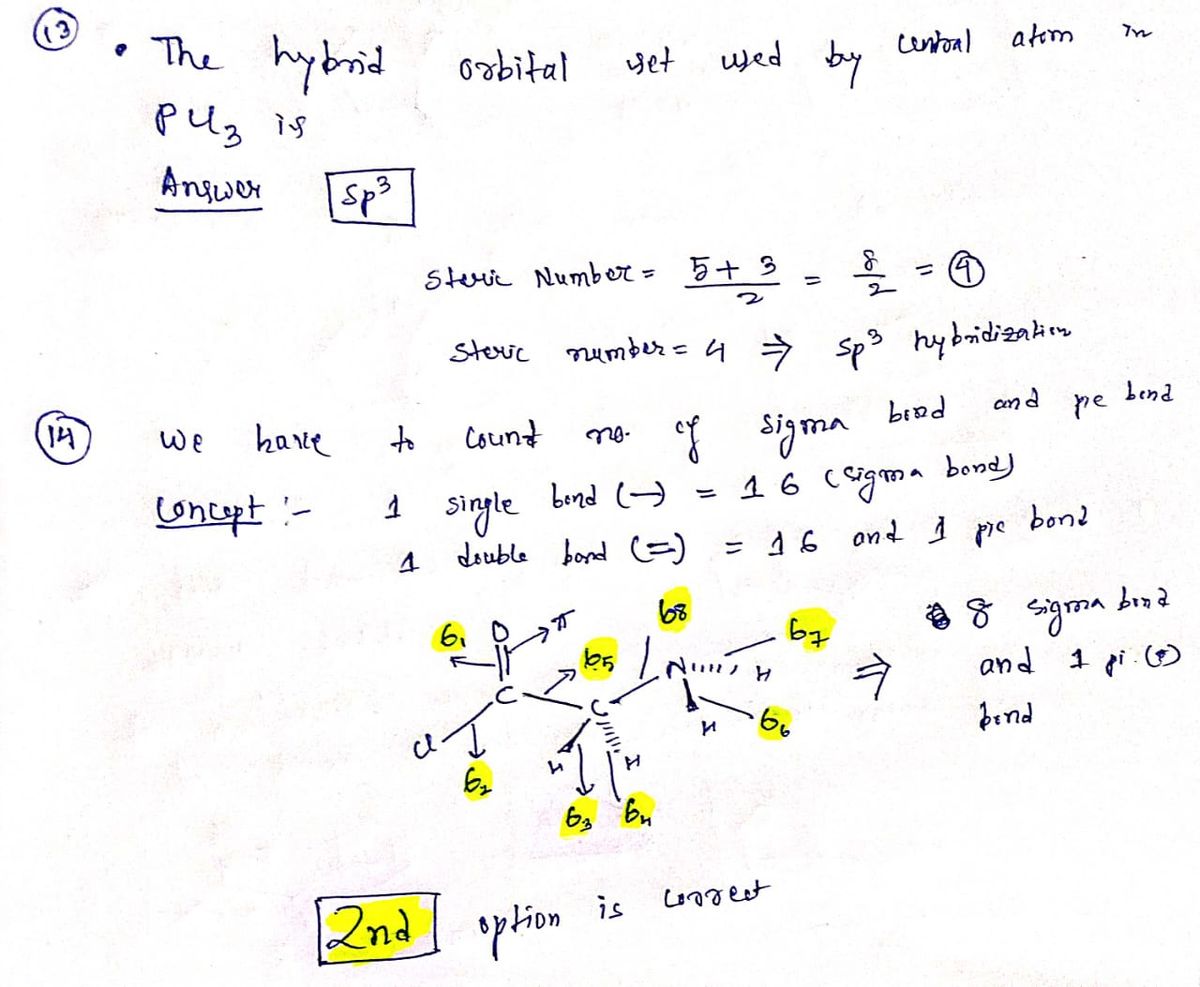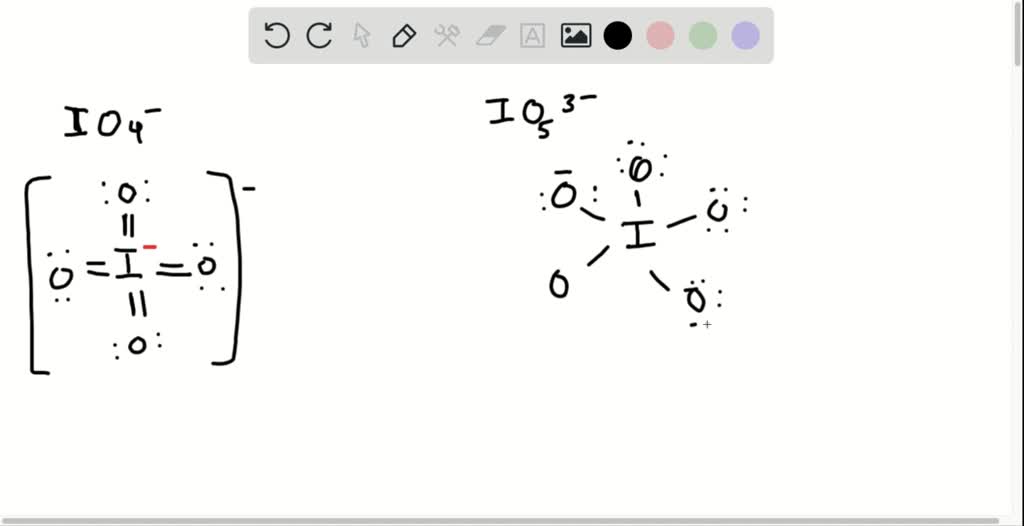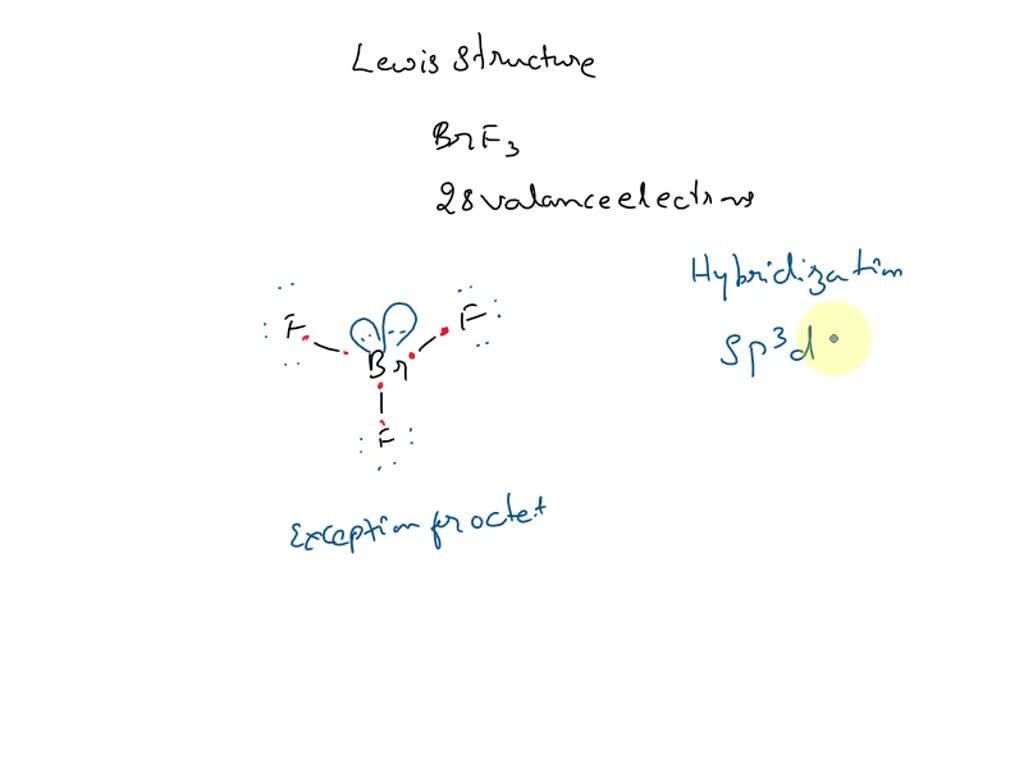
SOLVED: Draw a Lewis electron dot structure for each of the following. Show resonance structures where appropriate and show any formal charges. Give the molecular geometry and hybridization of the central atom.
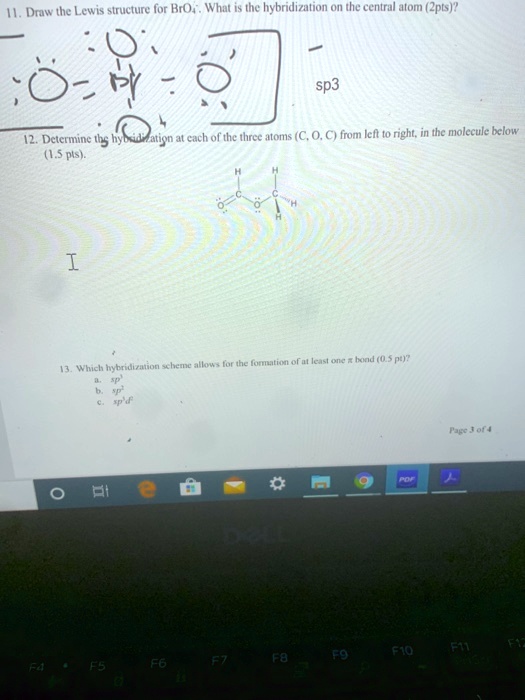
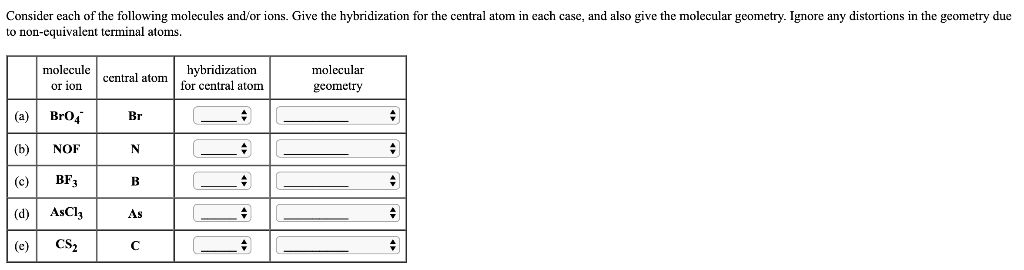
SOLVED: Draw the Lewis structure for BrO4-. What is the hybridization of the central atom (2p orbitals)? sp3 12. Determine the hybridization at each of the three atoms (C, O, E) from

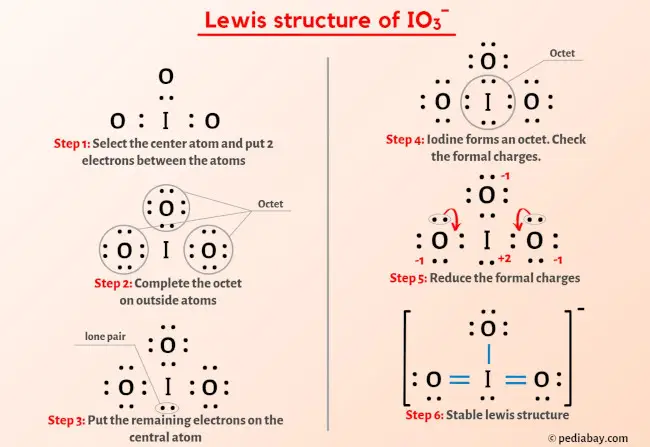
For the IO3-, a) Draw the electron dot diagram and structural formula b) Predict the shape c) Predict whether it is polar or non-polar, and justify your prediction. Indicate the positive and

In the Lewis structure of the iodate ion, IO3-, that satisfies the octet rule, what is the formal charge on the central iodine atom? | Homework.Study.com

Improvement of the Stability of IO3–-, SeO32–-, and SeO42–-Coprecipitated Barite after Treatment with Phosphate | Environmental Science & Technology
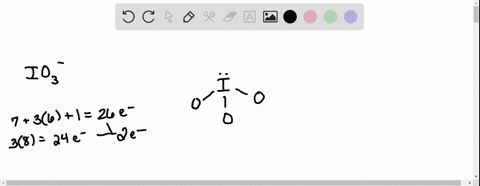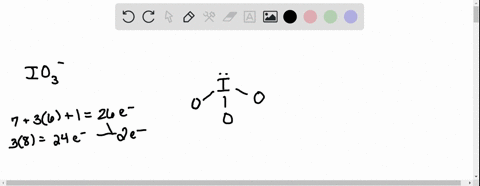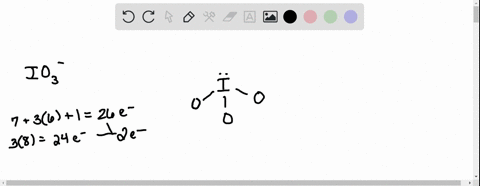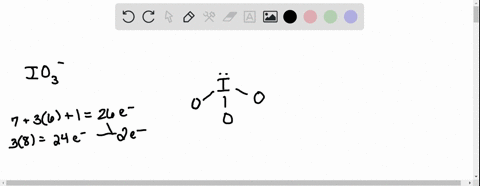
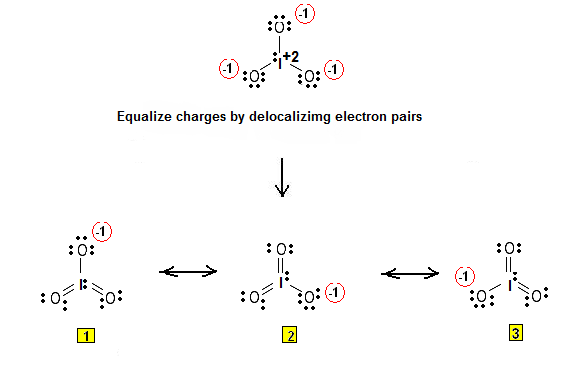
SOLVED: According to the principles of VSEPR theory applied on IO3- ion: a) Draw the most favorable Lewis structure with minimal formal charges (all O atoms are bonded to the central atom). Show all your work. b) Indicate the formal charges of all atoms. c ...

BiO(IO3): A New Polar Iodate that Exhibits an Aurivillius-Type (Bi2O2)2+ Layer and a Large SHG Response | Journal of the American Chemical Society
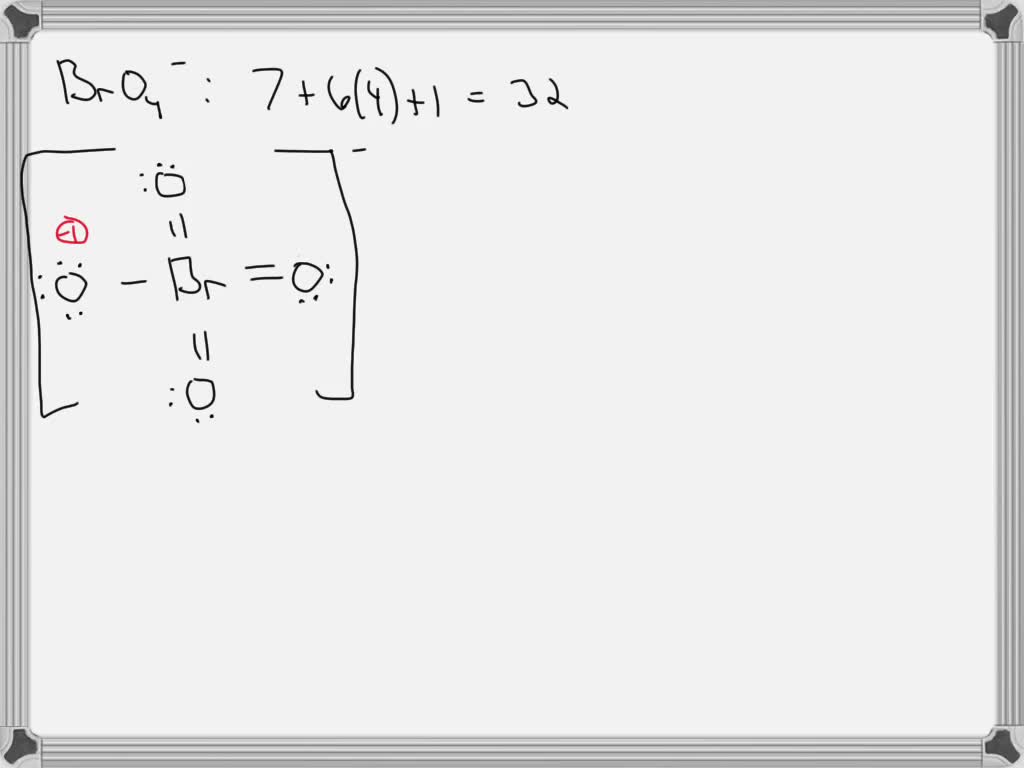
SOLVED: Draw the Lewis structure for BrO4-. What is the hybridization of the central atom (2p orbitals)? sp3 12. Determine the hybridization at each of the three atoms (C, O, E) from
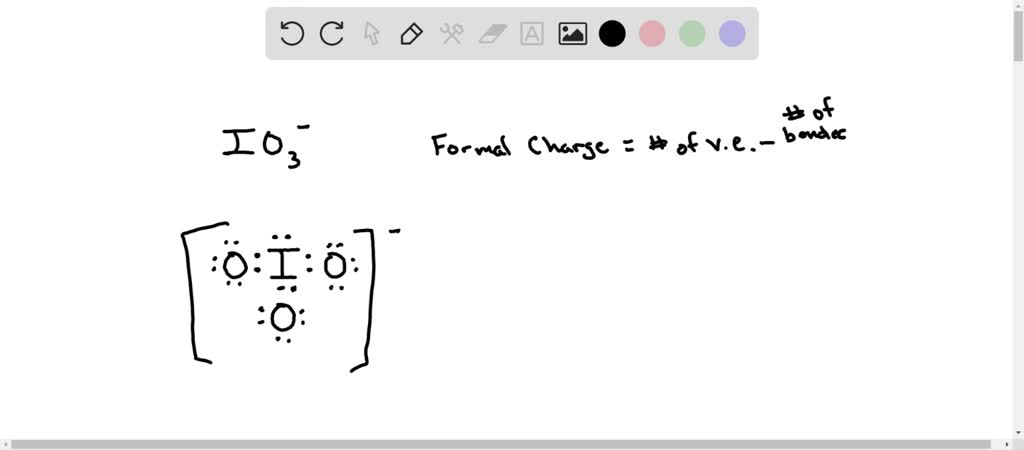
SOLVED: In the IO3- ion, iodine is the central atom. Based on the Lewis structure of IO3- that obeys to the Octet rule, what is the formal charge on the iodine atom?

Why the bond angle of XeO3 is 103 - Chemistry - Chemical Bonding and Molecular Structure - 12894693 | Meritnation.com