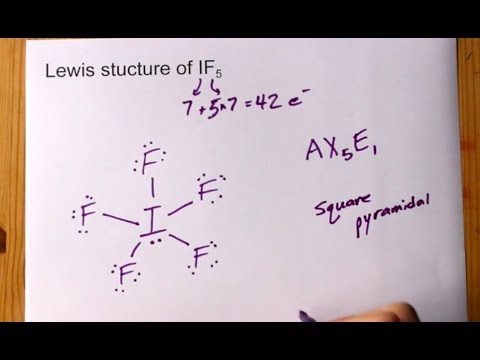
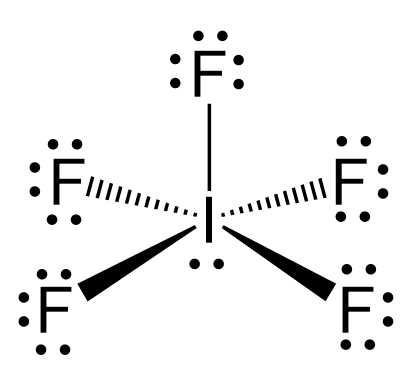
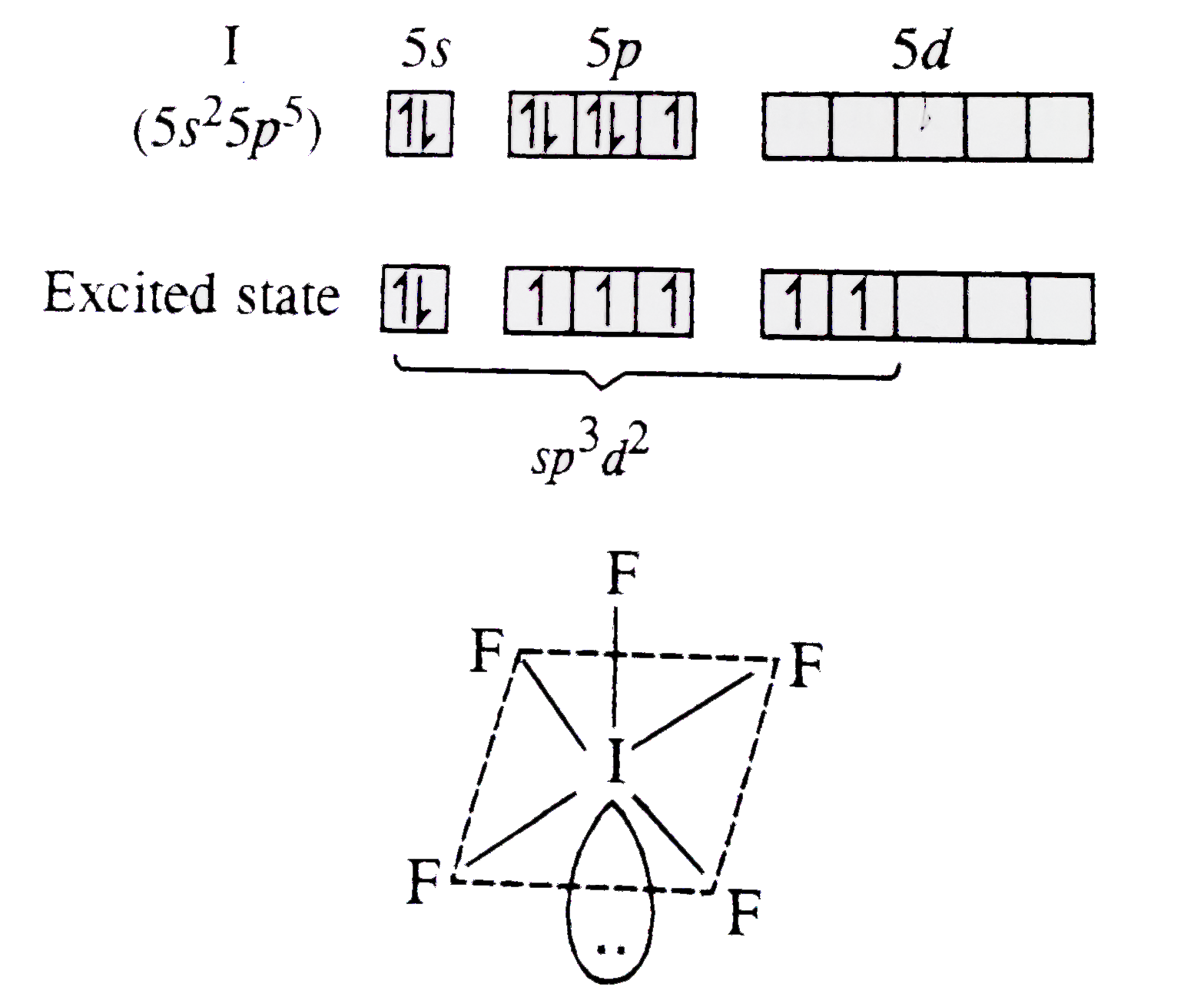Draw the Lewis structure for IF5 and answer the following questions. a. How many valence electrons are there? b. What is the electron geometry? c. What is the molecular geometry? d. What

Iodine pentafluoride IF5: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure –

The hybridization of P in POCl_3 and shape of POCl_3 are, respectively:sp^3, pyramidalsp^3, square planarsp^3, distorted tetrahedralsp^3, tetrahedral
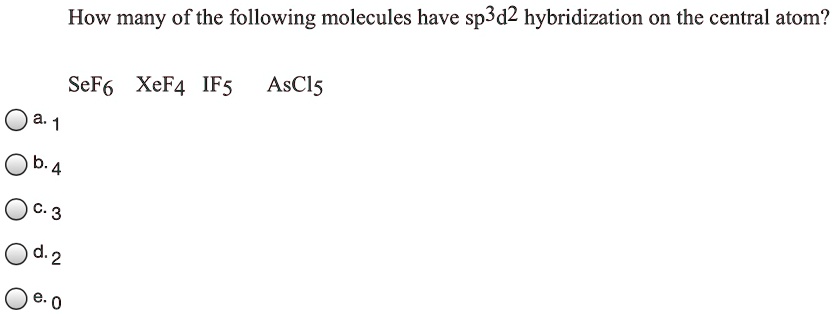
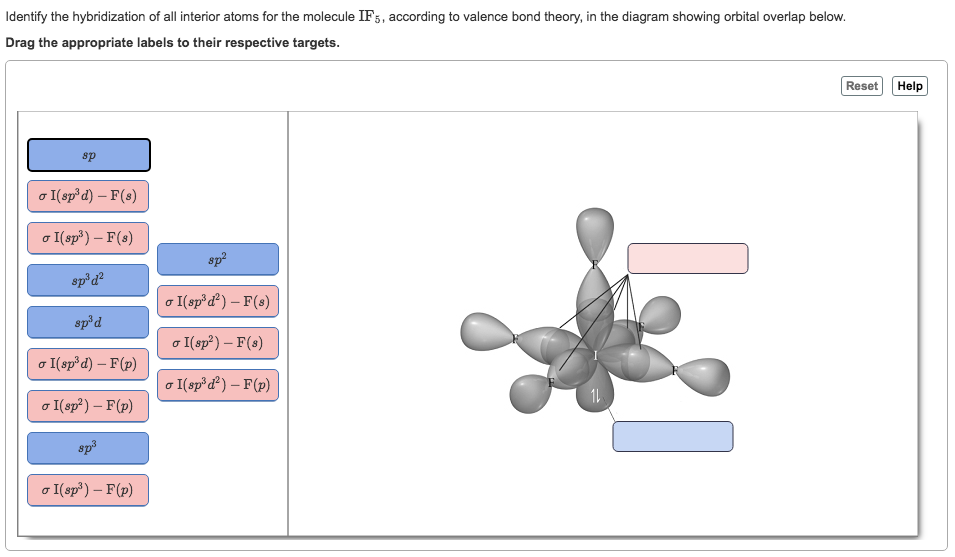
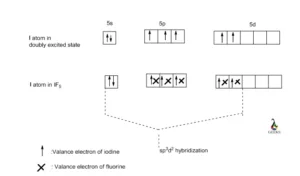
SOLVED: How many of the following molecules have sp3d2 hybridization on the central atom? SeF6, XeF4, IF5, AsCls a. 1 b. 4 c. 3 d. 2
![Shape and hybridization of \\[{\\text{I}}{{\\text{F}}_{\\text{5}}}\\] respectively are ______.A ) trigonal bipyramidal, \\[s{p^3}d\\]B ) see-saw \\[s{p^3}d\\]C ) square pyramidal, \\[s{p^3}{d^2}\\]D ) pentagonal pyramidal \\[s{p^3}{d^3}\\] Shape and hybridization of \\[{\\text{I}}{{\\text{F}}_{\\text{5}}}\\] respectively are ______.A ) trigonal bipyramidal, \\[s{p^3}d\\]B ) see-saw \\[s{p^3}d\\]C ) square pyramidal, \\[s{p^3}{d^2}\\]D ) pentagonal pyramidal \\[s{p^3}{d^3}\\]](https://www.vedantu.com/question-sets/14baae8b-19c1-486a-aeeb-fe0e96903d743424253312437900136.png)
Shape and hybridization of \\[{\\text{I}}{{\\text{F}}_{\\text{5}}}\\] respectively are ______.A ) trigonal bipyramidal, \\[s{p^3}d\\]B ) see-saw \\[s{p^3}d\\]C ) square pyramidal, \\[s{p^3}{d^2}\\]D ) pentagonal pyramidal \\[s{p^3}{d^3}\\]

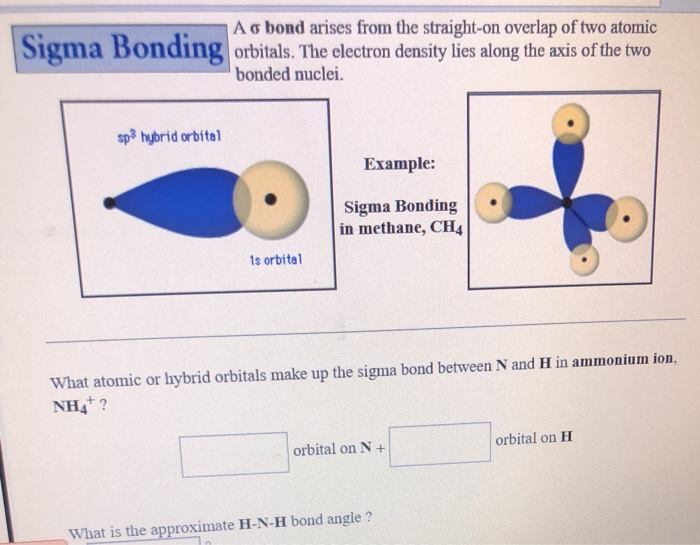
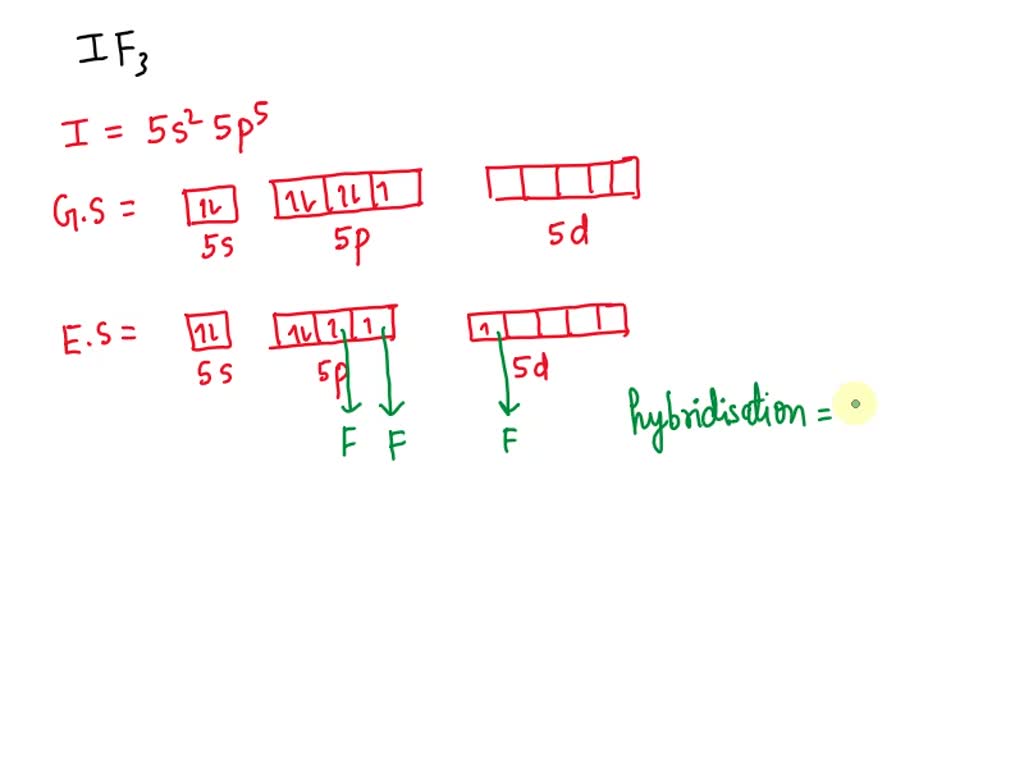
12. Which of the following compounds exhibit d 2sp 3 hybridization? (Select all that apply.) BrF5 ClF5 KrCl4 XeCl2 PCl5 13. Draw the Lewis structure for IF3 and answer the following question.