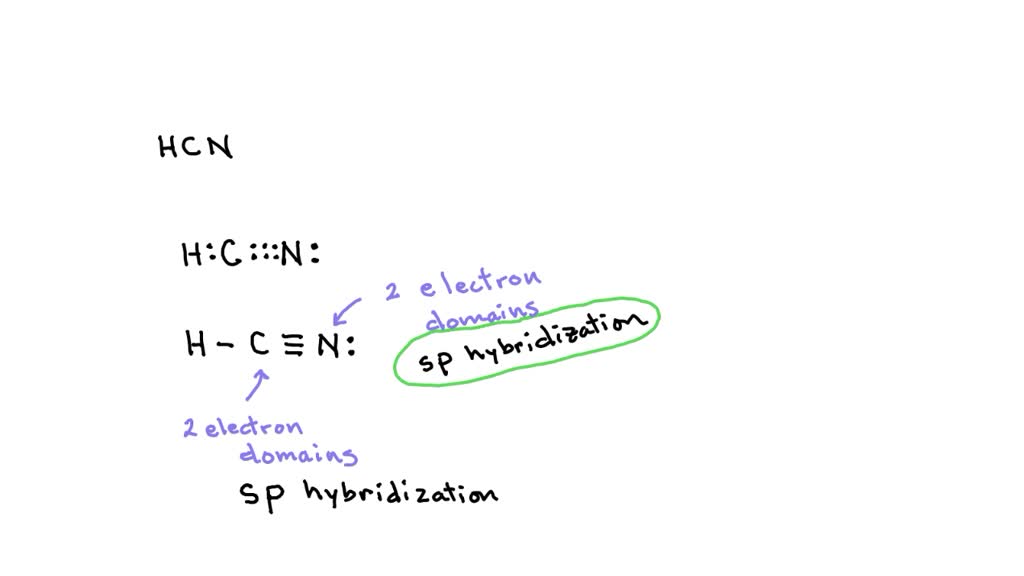
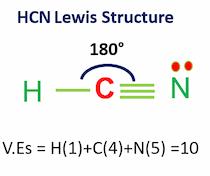
SOLVED: What is the hybridization of the central atom in each of the following: Drag the appropriate items to their respective bins. FeCl2 HCN TeCl2 SiCl4 SO2
Why does nitrogen in HCN hybridize, instead of forming 3 pi bonds? All 2p orbitals have only 1 electron. - Quora

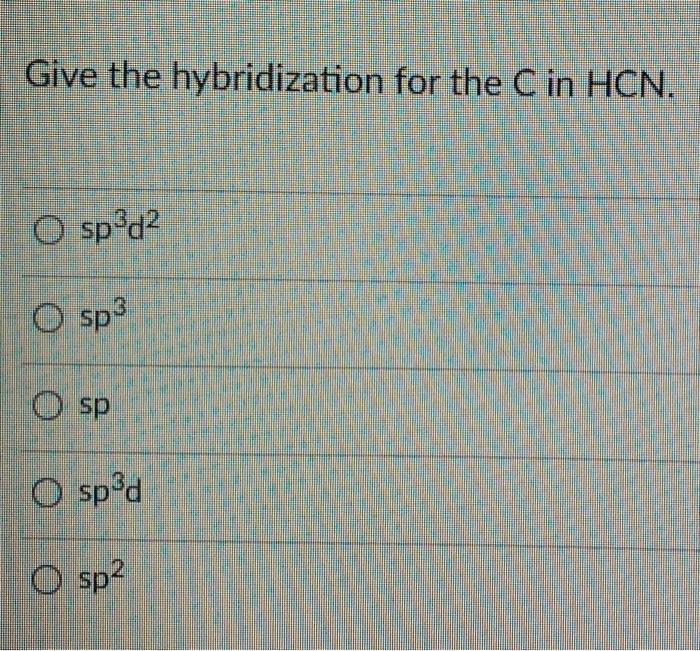
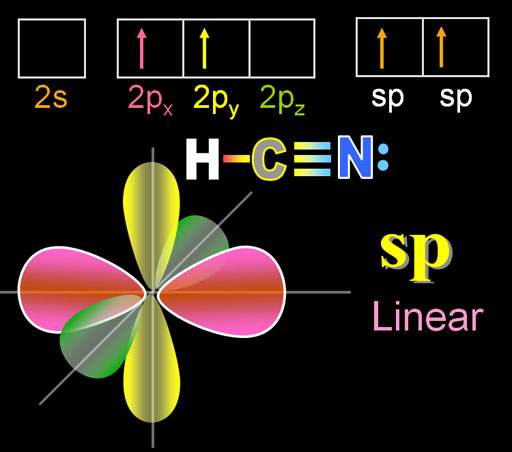
Solved) - What is the hybridization of the carbon in HCN? ?.sp B. sp C. sp3... (1 Answer) | Transtutors

Account for the hybridization at each carbon atom in the mentioned molecule. Chloroprene(used to make neoprene, a synthetic rubber) | Homework.Study.com

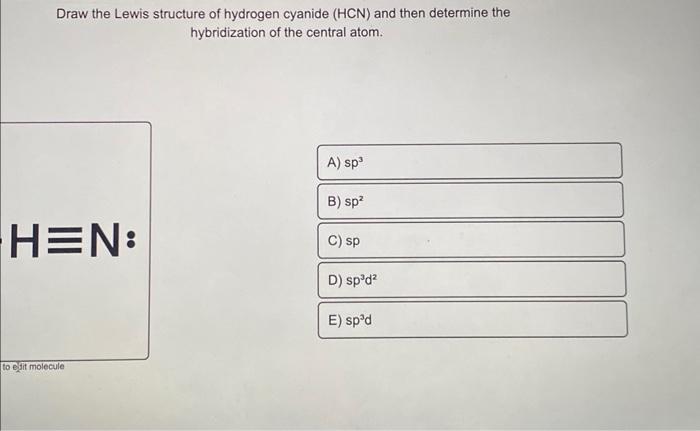
Which is the correct hybridization state and geometry for the carbon atom in HCN? a) sp, linear b) sp2, trigonal planar c) sp3, tetrahedral d) None of the above | Homework.Study.com