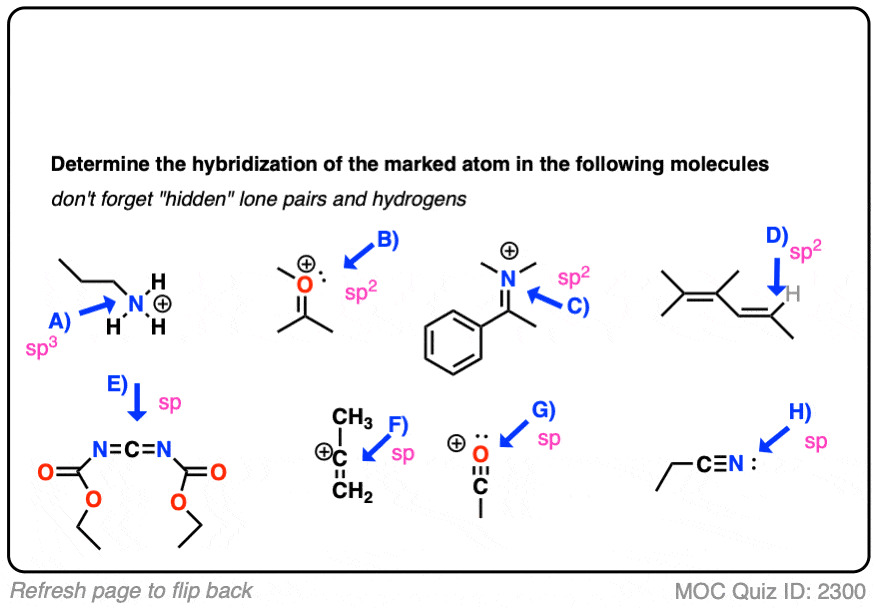
What is the hybridization of the carbon atom in each of the following CH_3^+, CH_3^-, CH_3? | Homework.Study.com
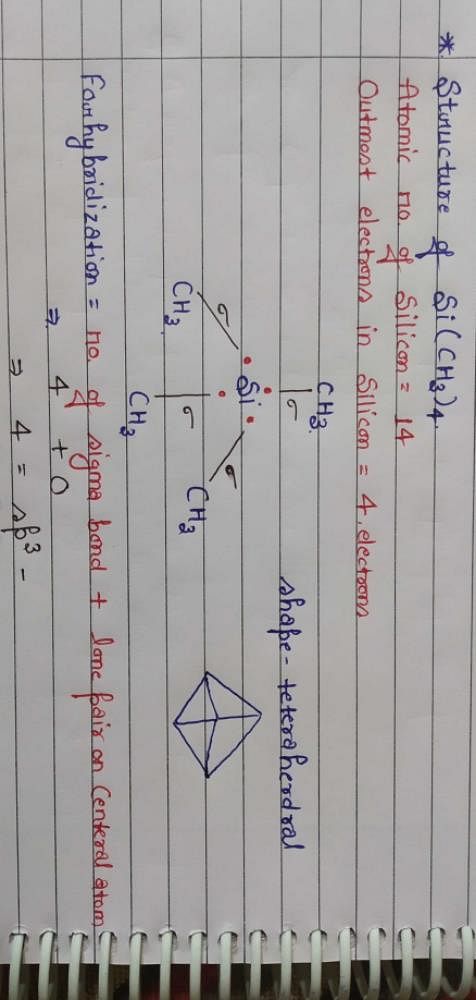
The structure and hybridisation of Si(CH3)4 isa)bent, spb)trigonal, sp2c)octahedral, sp3dd)tetrahedral, sp3Correct answer is option 'D'. Can you explain this answer? - EduRev NEET Question
10.What is the hybridization of Oxygen molecule in the following : 1. CH3 O CH=CH2 2. CH3COCH3 [Propanone]

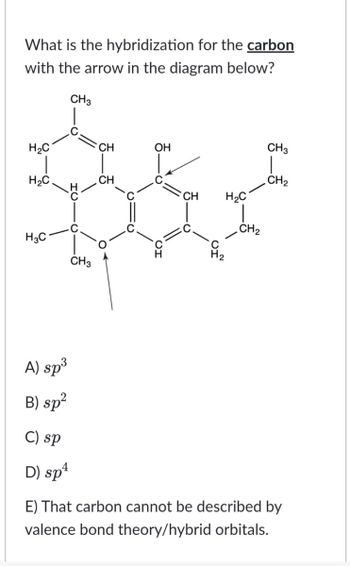
Hybridization of C2 and C3 of H3C andndash; CH = C = CH andndash; CH3 area)Sp, Sp3b)Sp2, Spc)Sp2, Sp2d)Sp, SpCorrect answer is option 'B'. Can you explain this answer? - EduRev JEE


![Solved] The hybridization of the central carbon in CH3C≡N and Solved] The hybridization of the central carbon in CH3C≡N and](https://storage.googleapis.com/tb-img/production/20/10/F1_Utkarsha_Madhu_20.10.20_D3.png)


![ANSWERED] What is the hybridization of the N in this amine? CH3-N-CH₂ - Kunduz ANSWERED] What is the hybridization of the N in this amine? CH3-N-CH₂ - Kunduz](https://media.kunduz.com/media/sug-question/raw/76146470-1659792437.8720496.jpeg)